
Colloidal microcrystalline cellulose (colloid MCC) is a versatile composite excipient widely used across industries. Composed of 80-90% microcrystalline cellulose (MCC) and 10-20% sodium carboxymethyl cellulose (CMC-Na), it forms a stable colloidal suspension or free-flowing powder. This unique combination enhances dispersibility, viscosity, and stability compared to pure MCC, making it indispensable in food, pharmaceutical, and industrial applications.
Key Properties:
Colloidal MCC’s unique properties drive its adoption in diverse sectors. Let’s explore how it adds value in each industry:
| Industry | Application Examples | Functional Role |
|---|---|---|
| Food | Dairy products, sauces, bakery, beverages | Thickener, stabilizer, fat replacer |
| Pharma | Tablets, capsules, controlled-release matrices | Binder, disintegrant, viscosity regulator |
| Cosmetics | Creams, lotions, gels | Rheology modifier, texture enhancer |
| Industrial | Paints, coatings, inks | Suspension aid, viscosity controller |
Why It Works:
Qingdao ACTA Biotechnology is China’s premier manufacturer of pharmaceutical- and food-grade colloidal MCC. Their products meet or exceed USP, EP, JP, and CP standards, backed by rigorous quality control.
ACTA offers tailored grades for specific applications. Below are the key specifications:
| TEST ITEMS | ACT591 | ACT3212 | ACT611 | ACT538 | ACT521 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 39-91 | 50-200 | 50-151 | 39-175 | 50-100 |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | food and beverage | Milk shake, sauce | food and beverage | Neutral milk drinks, vegetable protein drinks | food and beverage |
| TEST ITEMS | ACT509 | ACT600 | ACT610 | ACT428 | ACT631 | ACT651 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 60-150 | 2000-8000(2.6%) | 1200-2000 | 130-230 | 2000-8000(2.6%) | 50-151(2.6%) |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | Neutral milk drinks, vegetable protein drinks | Milk shake, sauce | Bakery,sauce | Neutral milk drinks, vegetable protein drinks | food and beverage | food and beverage |
3.
| TEST ITEMS | ACT440 | ACT450 | ACT480 | ACT996 | ACT981 | ACT8329 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 200-400 | 400-700 | 700-900 | 3000-5500(2.6%) | 2000-3500(2.6%) | 1000-1600(2.6%) |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | Paint and printing materials | Paint and printing materials | Bakery,sauce | food and beverage | food and beverage | food and beverage |
Extended Range:
ACTA employs advanced analytical methods to ensure product consistency:
| Test Parameter | Method | Standard |
|---|---|---|
| Viscosity | Brookfield viscometer (25°C) | Grade-specific ranges |
| Heavy Metals | Atomic Absorption Spectroscopy (AAS) | ≤10 mg/kg total |
| Microbial Safety | Plate count methods | Pathogen-free certification |
ACTA’s colloidal MCC is globally approved, ensuring safety and compliance:
Strict Limits:
ACTA offers competitive pricing without compromising quality:
| Source/Brand | Viscosity Range (mPa·s) | Price (USD/kg) | Notes |
|---|---|---|---|
| Qingdao ACTA | 39–2,000+ | 1.2–2.5 | Premium pharma/food grade |
| Roquette TABULOSE® SC 591 | 39–91 | 1.5–2.0 | Global benchmark |
| Sigma-Aldrich | Variable | 3.0–5.0 | Research-grade |
Qingdao ACTA Biotechnology’s colloidal MCC is ideal for:
In the specialized field of colloidal microcrystalline cellulose (CMC), Qingdao ACTA Biotechnology Co., Ltd. has emerged as a global industry leader through its technical expertise, uncompromising product quality, and strategic market vision. Since its establishment in 2002, the company has focused on research, development, and production of pharmaceutical-grade CMC, addressing the critical needs of domestic and international markets while redefining “Made in China” excellence in global biotechnology.
Qingdao ACTA Biotechnology understands that sustained technological innovation forms the cornerstone of competitiveness in the CMC sector. The company has continuously invested in R&D, imported advanced production equipment, and established a comprehensive system encompassing research, manufacturing, and quality control. Its CMC products exceed pharmacopoeia standards from Europe, the United States, and Japan across multiple critical performance indicators:
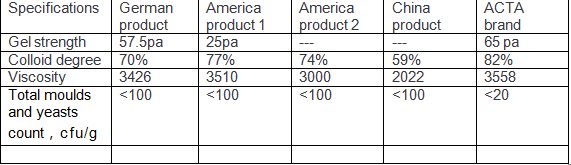
Proven Application Performance
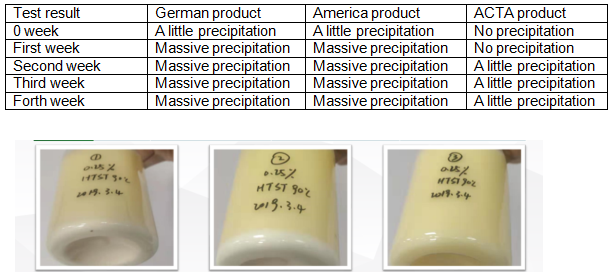
ACTA’s CMC products excel across multiple application domains:
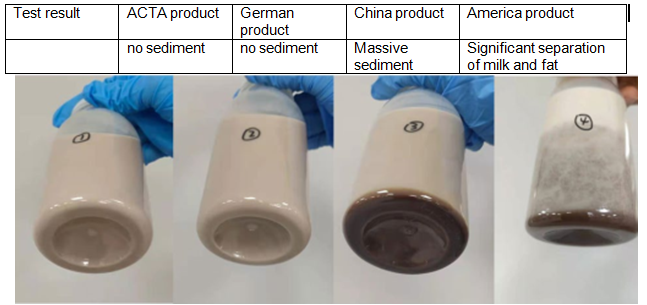
To continually enhance product quality and operational efficiency, ACTA has implemented a state-of-the-art smart manufacturing system:
Qingdao ACTA prioritizes customer success through:







For samples, technical datasheets, or formulation support:
References: