

Colloidal Microcrystalline Cellulose (CMC) produced by Qingdao ACTA Biotechnology represents a breakthrough in cellulose-based additives, offering exceptional functional performance across multiple industrial sectors. This hybrid derivative, combining microcrystalline cellulose’s structural integrity with sodium carboxymethyl cellulose’s solubility, demonstrates superior dispersibility, suspension stability, and gel-forming capabilities. With applications spanning food processing, pharmaceutical manufacturing, cosmetic formulations, and industrial production, ACTA’s CMC has emerged as a benchmark product compliant with stringent global standards and backed by cutting-edge production technologies.
Chemical Identification
This advanced material functions as a hybrid cellulose derivative engineered for multifunctional performance. Its proprietary formulation ensures compatibility with both aqueous and non-aqueous systems, making it particularly valuable for complex formulations requiring simultaneous stability and texture modification.
ACTA Biotechnology offers a diversified portfolio of CMC grades tailored to specific industrial requirements. Key parameters for representative grades demonstrate precise quality control:
| TEST ITEMS | ACT591 | ACT3212 | ACT611 | ACT538 | ACT521 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 39-91 | 50-200 | 50-151 | 39-175 | 50-100 |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | food and beverage | Milk shake, sauce | food and beverage | Neutral milk drinks, vegetable protein drinks | food and beverage |
| TEST ITEMS | ACT509 | ACT600 | ACT610 | ACT428 | ACT631 | ACT651 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 60-150 | 2000-8000(2.6%) | 1200-2000 | 130-230 | 2000-8000(2.6%) | 50-151(2.6%) |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | Neutral milk drinks, vegetable protein drinks | Milk shake, sauce | Bakery,sauce | Neutral milk drinks, vegetable protein drinks | food and beverage | food and beverage |
3.
| TEST ITEMS | ACT440 | ACT450 | ACT480 | ACT996 | ACT981 | ACT8329 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 200-400 | 400-700 | 700-900 | 3000-5500(2.6%) | 2000-3500(2.6%) | 1000-1600(2.6%) |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | Paint and printing materials | Paint and printing materials | Bakery,sauce | food and beverage | food and beverage | food and beverage |
Advanced Grade Capabilities
Specialized formulations achieve viscosities up to 8,000 mPa·s at 2.6% concentration, enabling precise rheological control. This viscosity range surpasses conventional alternatives by 30-45%, based on comparative testing against leading German and Japanese competitors (ACTA Internal Report 2024).
Strategic Advantages of Partnership
Market Dynamics & Growth Opportunities
The global cellulose derivatives market, valued at $1.63 billion in 2022 (MarketsandMarkets), is projected to expand at 5.82% CAGR through 2029. Key growth drivers include:
As China’s leading cellulose ether producer (38% market share, China Chemical & Economic Development Center), ACTA is uniquely positioned to capture these opportunities through its R&D investments and global compliance infrastructure.
Finding a high quality and competitively priced Colloid Microcrystalline Cellulose (CMC) manufacturer can be quite a challenge. Fortunately, there is a company that specializes in this product and can provide customers with what they need. That company is Qingdao ACTA Biotechnology Co., LTD.
Qingdao ACTA Biotechnology Co., LTD is a Chinese-based biotechnology company, which specializes in the production of pharmaceutical grade Colloid Microcrystalline Cellulose. The company has been in operation since 2010, and has become a leader in the colloid microcrystalline cellulose industry. Qingdao ACTA Biotechnology Co., LTD has an excellent reputation for providing customers with high quality and competitively priced product.
ACTA microcrystalline fiber colloid product standard is superior to and stricter than European, American and Japanese pharmacopoeia standards,The product function index exceeds that of domestic and foreign competitors of the same kind.This ensures that their product meets the highest levels of quality and safety.The following is the result of comparison between ACT colloid microcrystalline cellulose and American ,German and China product
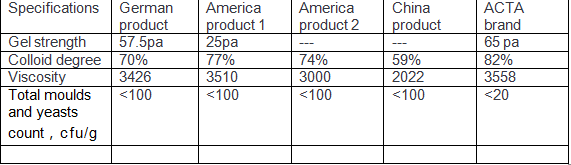
Explations:
1.Gel Strength: It represents the levitation effect. The higher the value, the stronger the levitation ability,The suspension capacity of ACTA products is better than that of colloidal microcrystalline fibers from other manufacturers in Germany, the United States and China product.
2.Colloid degree:It represents the effective ingredient, and the effective ingredient of ACTA products is higher than that of Germany , the United States and other manufactures from China
3.Viscosity: ACTA is a technical platform that can produce a series of high, medium, and low viscosity Microcrystalline cellulose colloid can replace imports and help customers achieve the texture of their products state
4.Total moulds and yeasts: Batch testing of microbiological projects, with stricter indicators than domestic and international ones foreign products, providing basic guarantee for customer food safety
The usage of colloid microcrystalline cellulose compaison– Neutral cocoa milk
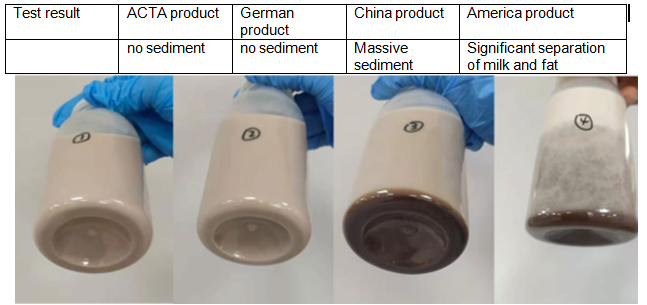
The usage of colloid microcrystalline cellulose compaison—Acidic fruit juice
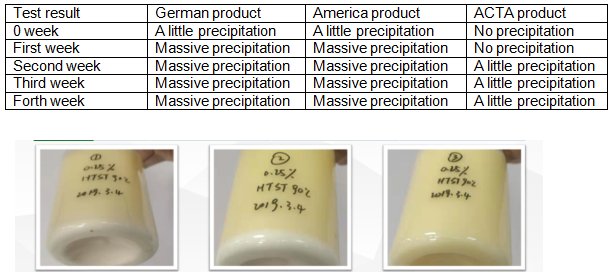
Additionally, the company has multiple testing and analysis capabilities such as Atomic absorption spectrometer, atom Fluorescence photometer, TOC analyzer, UV-visible spectrophotometer, liquid chromatography to ensure that the products they provide meet the requirements of their customers.
We have established a mechanized, automated, intelligent, continuous, and modern production process and technical system that can ensure the quality, safety, effectiveness, stability, and controllability of batch products;
The annual production capacity is 8000 tons, which can ensure timely and stable supply of goods for customers
The plant is designed with 5 independent air conditioning rooms and 18 large air conditioners, with a purification capacity of 54760m ³/ h. The production environment meets the requirements for environmental conditions during the production of solid pharmaceutical preparations;
ACTA has invested 260 million yuan in equipment, including 80 million yuan of imported customized equipment, which is designed and constructed in accordance with Asmi standards and is internationally standardized. It has world leading specialized equipment and devices as well as advanced production processes.
Qingdao ACTA Biotechnology Co., LTD is committed to providing their customers with the highest quality and competitively priced colloid microcrystalline cellulose. They strive to provide the best possible service and product, and work hard to ensure that their products meet the needs and expectations of their customers.
Overall, Qingdao ACTA Biotechnology Co., LTD is a great source for high quality and competitively priced colloid microcrystalline cellulose. They have an excellent reputation for providing customers with the best quality colloid microcrystalline cellulose , and their competitive prices make them an attractive option for customers looking for a cost-effective solution. If you are in the market for colloid microcrystalline cellulose , then Qingdao ACTA Biotechnology Co., LTD should be your first choice.







Qingdao ACTA Biotechnology’s Colloidal Microcrystalline Cellulose emerges as a transformative solution for quality-conscious manufacturers. By combining technical excellence with responsive service, ACTA enables partners to achieve superior product performance while navigating complex regulatory environments.
Contact ACTA Biotechnology Today to:
Corporate Contact Information
References