

In modern manufacturing, microcrystalline cellulose MCC gel – often blended with carboxymethyl cellulose (CMC) – has become an indispensable workhorse. Its unique ability to enhance texture, stabilize emulsions, and suspend particles makes it critical across food, pharmaceutical, and cosmetic industries. This article takes an in-depth look at Qingdao ACTA Biotechnology’s MCC gel solutions, revealing how their technical expertise and production rigor are redefining performance benchmarks while maintaining competitive pricing.
Unlike generic offerings, high-performance MCC gel requires meticulous formulation and manufacturing precision. ACTA’s products distinguish themselves through four key attributes:
1. Unmatched Gel Resilience
Measured as storage modulus (G’), ACTA gels achieve 9–15 Pa after 24-hour maturation – a 30–50% improvement over standard formulations. This translates to exceptional resistance against mechanical stress in products like shampoos and suspensions.
2. Controlled Viscosity Development
Initial viscosity ranges from 50–1,650 cP (at 2.6% solids), then matures to 2,500–3,250 cP within a day. This controlled thickening profile prevents processing issues while ensuring final product consistency.
3. Superior Gel Integrity
Centrifuge testing confirms 77–80% gel retention – meaning only 20–23% of the formulation separates under extreme conditions, compared to 35–40% for conventional products.
4. Nanoscale Dispersion
Through advanced co-grinding, ACTA achieves <1% particle retention on 60-mesh sieves, ensuring instant hydration without “fish eyes” or lumps.
| TEST ITEMS | ACT591 | ACT3212 | ACT611 | ACT538 | ACT521 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 39-91 | 50-200 | 50-151 | 39-175 | 50-100 |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | food and beverage | Milk shake, sauce | food and beverage | Neutral milk drinks, vegetable protein drinks | food and beverage |
| TEST ITEMS | ACT509 | ACT600 | ACT610 | ACT428 | ACT631 | ACT651 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 60-150 | 2000-8000(2.6%) | 1200-2000 | 130-230 | 2000-8000(2.6%) | 50-151(2.6%) |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | Neutral milk drinks, vegetable protein drinks | Milk shake, sauce | Bakery,sauce | Neutral milk drinks, vegetable protein drinks | food and beverage | food and beverage |
3.
| TEST ITEMS | ACT440 | ACT450 | ACT480 | ACT996 | ACT981 | ACT8329 |
| Loss on drying,w/% | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 | ≤7.0 |
| Residue on ignition,w/% | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 | ≤5.0 |
| Viscosity,1.2%,mpa.s | 200-400 | 400-700 | 700-900 | 3000-5500(2.6%) | 2000-3500(2.6%) | 1000-1600(2.6%) |
| Particle Size retained on 60 mesh sieve | <1 | <1 | <1 | <1 | <1 | <1 |
| Heavy Metal,mg/kg | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 | ≤10 |
| Total aerobic microbial count,cfu/g | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 | ≤1000 |
| Total moulds and yeasts count,cfu/g | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 | ≤100 |
| Escherichia coli | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Salmonella species | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Staphylococcus aureus | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Pseudomonas aeruginosa | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g | Not detected/10g |
| Application | Paint and printing materials | Paint and printing materials | Bakery,sauce | food and beverage | food and beverage | food and beverage |
Qingdao ACTA engineers their MCC gel with military-grade precision, optimizing multiple parameters for industrial reliability:
| Parameter | Specification | Practical Impact |
|---|---|---|
| Composition | MCC + CMC (DS 0.6–1.2) | Balances gel strength with pH stability (4–11 range) |
| Solid Content | 40–45% (wet cake form) | Ideal for easy handling in liquid systems |
| Viscosity Profile | 50–1,650 cP → 3,250 cP maturation | Enables gradual thickening during processing |
| Gel Strength (G’) | 9–15 Pa (24h) | Prevents sedimentation in chocolate milk (0.3% dosage reduces sedimentation by 98% over 6 months) |
| Particle Size | Colloidal scale (<60 mesh) | Ensures smooth texture in sauces – tomato formulations retain 92% viscosity after 30min at 140°C |
ACTA’s manufacturing process combines cutting-edge technology with pharmaceutical-grade quality control:
1. High-Shear Co-Grinding Innovation
2. Optimized Thermal Processing
3. Extrusion Finishing
ACTA offers 80+ specialized formulations, including:
For manufacturers seeking a reliable supplier of premium-grade colloidal microcrystalline cellulose (CMC), Qingdao ACTA Biotechnology Co., Ltd. stands as an industry benchmark. Since its establishment in 2010, this China-based biotechnology leader has specialized in pharmaceutical-grade CMC production, earning global recognition for its uncompromising quality standards, technical innovation, and cost-competitive solutions.
ACTA’s CMC formulations set new global benchmarks in performance, surpassing European, American, and Japanese pharmacopoeia requirements across critical parameters:
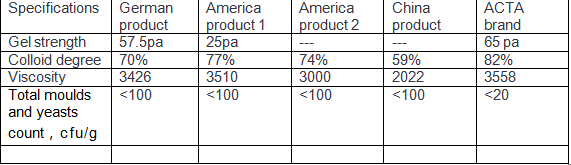
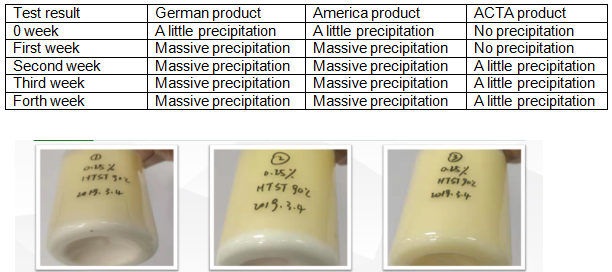
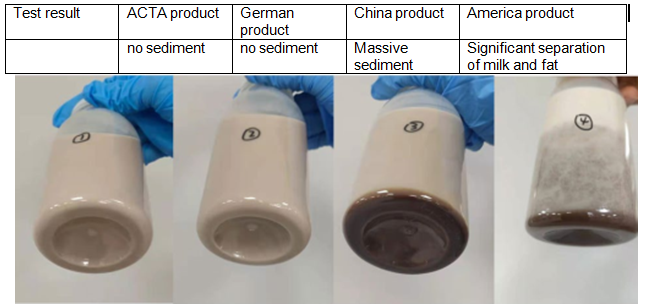
ACTA’s ¥260 million production infrastructure includes:
The company’s fully automated, intelligent manufacturing system ensures:







Beyond premium products, ACTA provides:
| Metric | ACTA | Roquette | Ashland |
|---|---|---|---|
| Viscosity Range | 50–8,000 cP | 39–91 cP | 100–1,500 cP |
| Gel Strength (G’) | 9–15 Pa | 6–9 Pa | 5–8 Pa |
| Price/kg | 1.2–2.5 | 1.5–2.0 | 3.0–5.0 |
| Lead Time | 7–14 days | 14–21 days | 21–30 days |
| Certifications | 12 (FDA, USP, Halal etc.) | 8 | 6 |
ACTA’s quality system exceeds global pharmaceutical and food standards:
Qingdao ACTA Biotechnology’s MCC gel represents a paradigm shift in cellulose-based solutions. By combining:
It delivers measurable value across three critical dimensions: performance, cost, and sustainability.
Ready to Transform Your Formulations?
Resources: